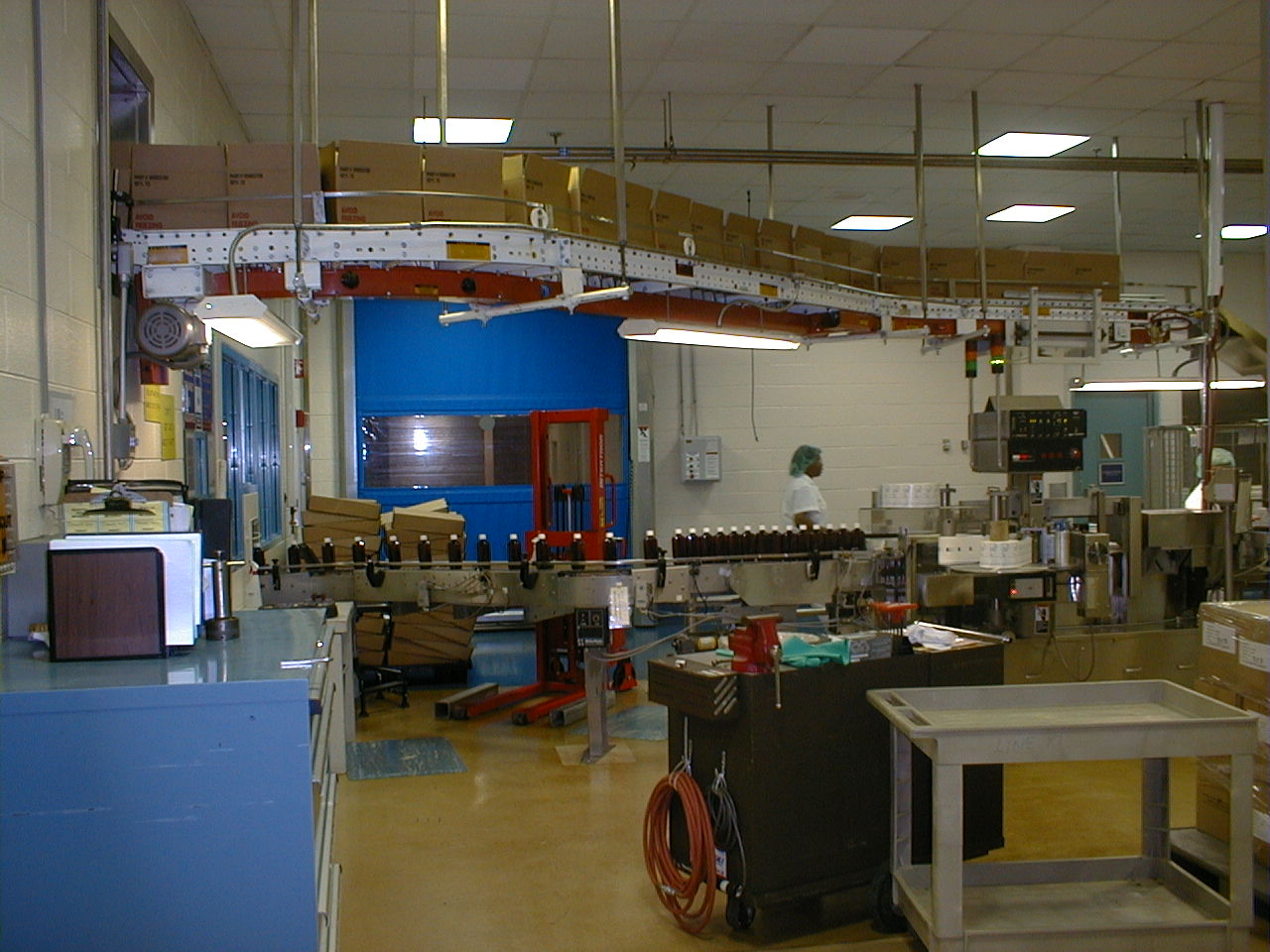
The space consisted of a high-level clean room and required that everyone involved in the project be dressed in a gown when entering the facility. All rooms constructed were explosion rated. The work took place in numerous areas of the plant, both inside and out, while all other areas continued to operate and manufacture other products with no interruption.
Construction included the installation of new chemical storage and feed systems, solution piping, dust collection systems for product ingredients, and necessary solvent and aqueous equipment; as well as renovations to the associated spaces. The project included relocation and installation of the field equipment packaging lines within the working facility; the construction and renovation did not impact production. Spaces were modified for new distribution and packaging for all products.


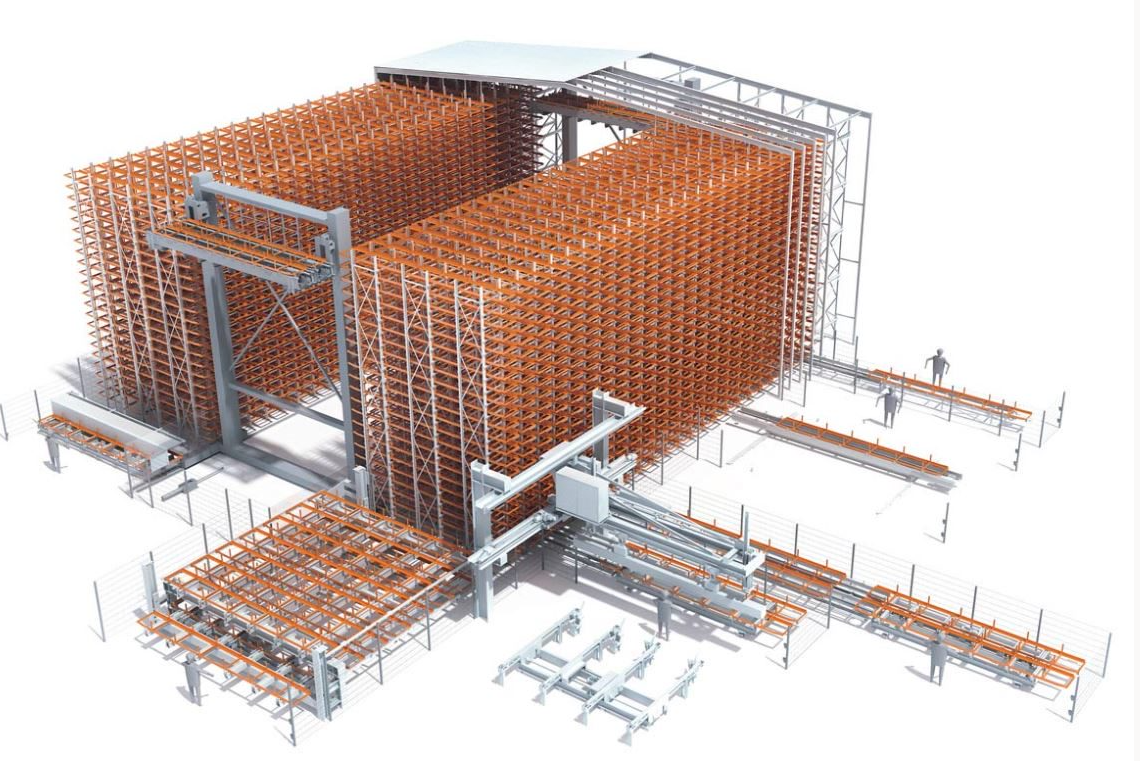
Work also included the relocation of process spaces, requiring new facilities. Six million dollars of the work was completed over a 12-week period within active production areas. All work took place in and around day-to-day activities of the plant including product production.


The pharmaceutical facility was the largest of 18 to 20 projects completed by McCownGordon over a 3-year time span. Projects were completed under construction manager and design-build delivery methods. Projects in addition to this project ranged in size from $100,000 to $4 million and consisted of renovating process facilities, addition of clean rooms and air locks, expansion of existing services and production facilities. All work was completed with minimal to no interruption of day-to-day plant activities.